

Our Mission at GNT Animal Health
We aim to provide products that go beyond merely treating and controlling animal diseases.
GedaCure
Product
Information


Appearance
Brown-colored round chewable tablet
Active Ingredient
Crisdesalazine
Indications
Control of clinical signs associated with canine cognitive dysfunction syndrome
Dosage / Administration
Refer to the labelling (attached file) and administer the pill once a day according to the body weight.
Storage
Avoid direct sunlight and store at room temperature (1~30℃).
Packing
30 Tablets / package
What is GedaCure®?
GedaCure® is the world's first canine cognitive dysfunction syndrome treatment.
The name is a combination of Gedächtnis, a German word meaning - "memory".
Also, Cure an English word meaning - "healing or treating".
The name is a combination of Gedächtnis, a German word meaning - "memory".
Also, Cure an English word meaning - "healing or treating".
As the number of households with companion animals grows, advancements in veterinary medicine have led to increased life expectancy for dogs each year.
With this longer lifespan, cases of Canine Cognitive Dysfunction Syndrome have also risen, prompting owners to pay more attention to their dogs.
With this longer lifespan, cases of Canine Cognitive Dysfunction Syndrome have also risen, prompting owners to pay more attention to their dogs.
GedaCure® is a new treatment that addresses cognitive dysfunction syndrome in dogs by effectively
reducing oxidative stress and inflammation in the brain, thereby preventing brain cell death.
reducing oxidative stress and inflammation in the brain, thereby preventing brain cell death.
Active Ingredient
Crisdesalazine
Crisdesalazine, the active ingredient in GedaCure®, is a novel drug with dual pharmacological mechanisms: powerful antioxidant and anti-inflammatory actions. It has been shown to enhance cognitive function by effectively reducing oxidative stress and inflammation in the brain, thereby protecting neurons in transgenic mouse models of Alzheimer’s disease.


Crisdesalazine reduces oxidative stress in neuronal cells
by removing hydroxyl radicals.
by removing hydroxyl radicals.


Crisdesalazine is an mPGES-1 inhibitor that reduces inflammation
in the brain by inhibiting PGE2 synthesis.
in the brain by inhibiting PGE2 synthesis.
Shin et al. Concurrent blockade of free radical and microsomal prostaglandin E synthase-1-mediated PGE2 production improves safety
and efficacy in a mouse model of amyotrophic lateral sclerosis. J Neurochem 2012; 122(5): 952-961
and efficacy in a mouse model of amyotrophic lateral sclerosis. J Neurochem 2012; 122(5): 952-961


After daily oral administration of crisdesalazine at 200 mg/kg (40 times the recommended dose for dogs) for 13 weeks, no significant differences were found between the crisdesalazine-treated dogs and the normal control group in vital signs (blood pressure, heart rate, etc.), electrocardiogram, ophthalmic examination, complete blood cell count, serum chemistry, urinalysis, or histological examination of other organs.
Additionally, no significant abnormalities were observed in our phase III clinical trial for CCDS approval, where similar tests were conducted on dogs.
In a comparison of gastrointestinal side effects following high doses of crisdesalazine and several NSAIDs (representative anti-inflammatory drugs) in rats, those treated with aspirin, ibuprofen, or celecoxib showed significant gastrointestinal bleeding. In contrast, rats treated with crisdesalazine, even at a dose of 1000 mg/kg (200 times the recommended dose), showed no gastrointestinal side effects.


Crisdesalazine
1000 mg/kg
-

 Aspirin300 mg/kg
Aspirin300 mg/kg -

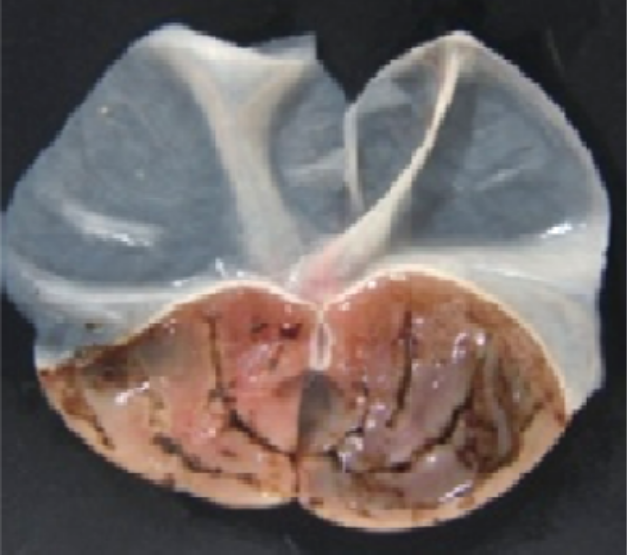 Ibuprofen300 mg/kg
Ibuprofen300 mg/kg -

 Celecoxib1000 mg/kg
Celecoxib1000 mg/kg
Brand Identity


01
Meaning “a fundamental treatment”
by placing “Cure” in the center.
02
Meaning “cure the memory” by
connecting the C (Cure) from the G
(Gedächtnis, memory)
03
The overall shape is a combination of
a circle and a straight line away from a
formalized circle, with a repetitive and
stable pattern.